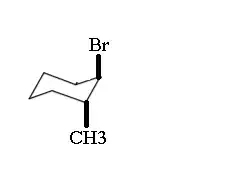
The question is asking for the product and the answer is C. My expected answer was E. Here's how I tried to solve it: Since Br (the leaving group) is at the equatorial position, the structure would 'ring flip'.
Since $\ce{CH3}$ is pointing downwards, I assumed it would have methyl group point outwards (dashed), however, the correct answer is a wedge.
After elimination, how would we know whether the substituent would be pointing inwards, outwards, or even right on the plane? (no wedge or dash)