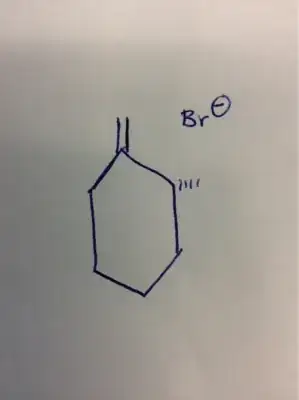
If the elimination occurs via an ${\mathrm{E_2}}$ mechanism, the other endocyclic double bond (i.e., the one between the two methyl bearing carbons) cannot be formed, as the $\ce{C_2}$ $\beta$-hydrogen will not be anti-periplanar to the $\ce{Br-}$ leaving group. That said, the other possible elimination product you've identified, with the exocyclic double bond, is entirely possible. However, this would be the Hofmann product, as it contains the less-substituted double bond. The major product of an elimination reaction is typically the Zaitsev product, which contains the most-substituted double bond. The Zaitsev product is the thermodynamic one (as the more-substituted alkene is typically more stable), while the Hofmann product is the kinetic one (as it results from a base abstracting a more sterically accessible or otherwise more acidic hydrogen). The Hofmann product can be obtained if a sterically bulky base is used, as this raises the energy of the transition state due to steric hindrance between the approaching base and the substrate molecule, and puts the reaction under kinetic control (assuming reaction temperature is controlled such that the $E_a$ for the thermodynamic pathway is prohibitively high). $\ce{NaOEt}$ is not bulky, so we expect the Zaitsev product (as given in the reaction scheme you cite).
If an $\mathrm{E_1}$ mechanism occurs, a tertiary carbocation intermediate is generated, and the double-bond is likely to form between the two methyl bearing carbons, as this would give the maximally stable tetrasubstituted alkene, and the requirement for anti-periplanarity is lifted. That said, $\mathrm{E_1}$ reactions are not favorable given a high concentration of strong base, so I would expect $\ce{E_2}$ to predominate, assuming at least a stoichiometric amount of $\ce{NaOEt}$ is present.
(Note that, with cyclohexanes, the issue is slightly more complex, as the anti-periplanarity requirement of the $\mathrm{E_2}$ mechanism requires the leaving group to be axial, which in turn necessitates a ring flip that has an associated energy barrier. Hence, in theory, the reaction with a cyclic molecule may be more likely to be kinetically controlled under a wider range of conditions.)