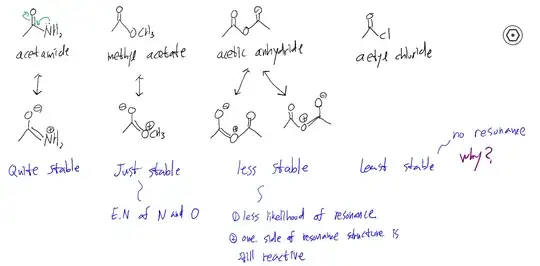
This picture depicts the relative stability associated with resonance structure.
My questions are:
1.1. Teacher says anhydride is less stable because of two reasons above.
but it seems there's no reason the resonance of anhydride would be less likely compared to acetate.
1.2. and about reason #2, the actual structure is the hybrid of these resonances so it's hard to agree with this reason.
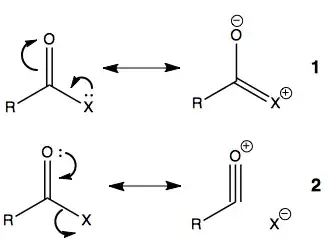
- Why won't Cl give its e- pair to adjacent carbon for resonance structure.
Someone says it's about electronegativity but it contradicts with resonance associated with oxygen. And someone says it's about the high energy orbital of Cl (n=3) but I can imagine a acetyl fluoride and this logic doesn't solve this problem anymore.