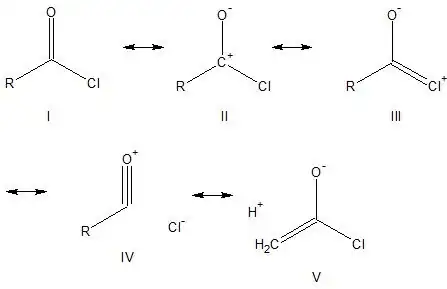
I know that in acyl chlorides, there is partial triple bond character in the $\ce{C=O}$ bond not because the chlorine is electronically donating (due to size mismatch with carbon) but because the chlorine is highly electron withdrawing and this stimulates the oxygen into partially donating one of its lone pairs to the partially positive carbon.
This is supported by IR stretch data for the $\ce{C=O}$ bond in acid chlorides; we're looking at frequencies from 1810-1775 reciprocal centimeters - somewhat higher than a vanilla $\ce{C=O}$ bond stretch frequency.
However, in acid fluorides, in which the fluorine is of comparable size to the central carbon, it seems that resonance donation is at least a possibility. However, resonance must compete with the inductive withdrawal effect. So, which one wins out in acid fluorides, and does the $\ce{C=O}$ bond exhibit more single bond character or triple bond character?
I tried looking up acid fluorides, and I can't find any mention of them. So I guess a good starting point would be: are they stable enough to even exist?