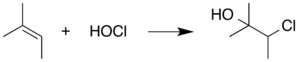I have read that hypohalous acids react with alkene to give halohydrin. For example the below reaction of hypochlorous acid with 2-methyl-2-butene which I found in Wikipedia.
Now my question is: Since hypochlorous acid is an acid it should give $\ce{H+}$ and $\ce{OCl-}$ ions which it does according to Wikipedia (although it is a weak acid), so shouldn't it add as $\ce{H+}$ and $\ce{OCl-}$ like other hydrogen halides add to alkene? Why would it add as $\ce{HO-}$ and $\ce{Cl+}$?