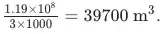Some companies are refitting steam power stations to hot basalt, where many tons of rocks are heated to 750°C by wind power and the thermal energy is used to generate steam in power stations.
How much basalt can supply 11,000 megawatt-hours, enough to power New York City for a day, given a total process efficiency rate of 55%?