I have learnt that at room temperature there are some free electron(not bound to any nucleas) in a conductor and when an electric field is applied they form an electric current.I am quite comfortable with this theory but then I am introduced to band theory which talks of valence and conduction band and they write when electrons can jump from valence to conduction band they conduct electricity.I cannot get what these bands are and why that jump causes current flow?I think the energy level of the valence electrons of all the atoms in the crystal form valence band(correct me,if wrong) but I have no idea about conduction band.Is it allowed energy levels of free electrons present in the crystal or something else?Please clarify
-
You should distinguish conductors from semiconductors. – my2cts Jan 30 '20 at 07:46
-
@Photon Actually both questions are mine only and there is a clear difference between them – Sharad1 Jan 30 '20 at 07:55
-
There have already been many answers to your questions. Please clarify what part exactly is troubling you. – Superfast Jellyfish Feb 20 '20 at 11:05
2 Answers
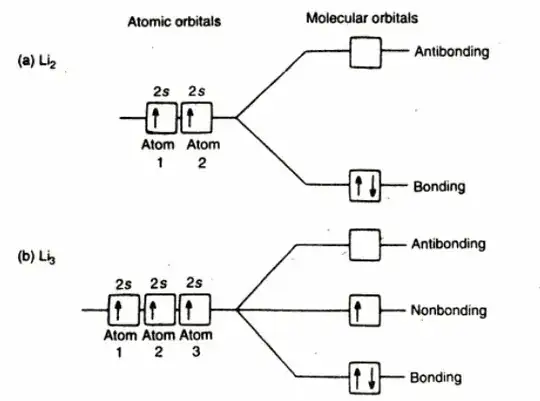
The band model is based on the molecular orbital theory:
According to this model, roughly what happens is that atomic orbitals mix up to form each time two molecular orbitals: one is called the bonding M.O., the other is the *antibonding M.O. The first promotes the formation of a bond between molecules, the second opposes. The bonding M.O.'s are filled before than the others for each two groups of energetic sublevels which mix together.
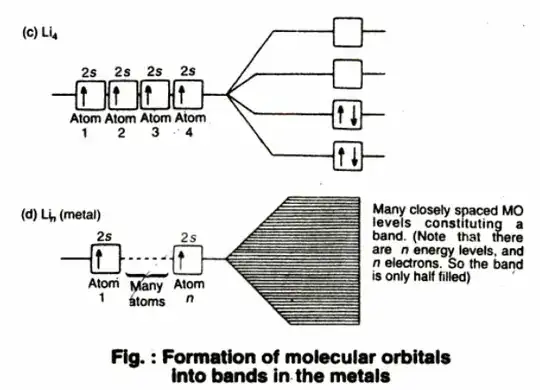
In a metal, many atoms form together the so-calles metallic bonding, and for each atom there are additional bonding and antibonding molecular orbitals. In a few grams of a metal such as Lithium, there are around $10^{23} $ atoms. When there is exceedingly large number of atomic orbitals, the energy separation between two any levels would be very small and a continuous energy band is obtained.
A metallic lattice has an extremely large number of atoms. The atomic orbitals of these atoms overlap resulting in the almost continuous energy band having large number of energy levels. Each energy level in the bond of Lithium can accomodate two electrons. The molecular orbitals extend in three dimensions over all the atoms in the crystal, so electrons have a high degree of mobility. These mobile electrons account for the high thermal and electrical conduction by metals.
When one end of the metal is heated, electrons at that end acquire energy and move to an unoccupied part of the energy band, where they can travel rapidly to any other part of the energy band, which in turn becomes hot. In a similar manner electric conduction takes place through a minor perturbation of energy promoting some electrons to an unfilled level, where they can move rapidly.
The conduction (in a metal) occurs because the molecular orbitals extend over the whole crystal and because there is no energy gap between filled and vacant molecular orbitals. The absence of energy gap in lithium is because only half the molecular orbitals are filled with electrons.
In case of Beryllium, the situation is slightly different:
In general, the energy gap in metals is so small, that even a little quantity of energy (a perturbation somehow) succeeds in promoting several electrons towards the antibonding orbitals - the conduction band. This perturbation is already present due to temperature, but the thermal energy is small compared to the electric flow that an external electric field, a beam of light tuned at a certain frequency, or other can provoke in the metal.
Then there are also semiconductors and insulators, for which the energy gap is respectively medium and large, but this is another story...
-
"This perturbation is already present due to temperature, but the thermal energy is small compared to the electric flow that an external electric field". Do you mean that even without external electric field we have current due to thermal excitation? – Anton Mar 14 '21 at 17:45
-
1@Anton Correct, in the sense that some electrons are free to move. However, due to their random motion, the net overall current is likely to be zero. – Shootforthemoon Apr 07 '21 at 17:18
-
How the electrons the conduction band electrons are replenished? Also if we were "hitting" the metal with photons of appropriate energy could we make the current bigger? – Anton May 07 '21 at 11:58
Solids contain a huge no of atom packed closely together, when such a atom is isolated then it is discrete set of electronic energy levels and when the isolated atom brought together their outermost electronic level overlap and the form energy bands to preserve the Pauli exclusion principle.
When the distance between atoms approaches the equilib- rium, this band splits into two bands separated by an energy gap Eg. The upper band is called as the conduction band . And the lower one is called valence band. Thus, apart from the low-lying and tightly bound “core” levels, the crystal has two bands of available energy levels separated by an energy gap Eg wide, which contains no allowed energy levels for electrons to occupy. This gap is sometimes called a “forbidden band".
https://en.m.wikipedia.org/wiki/File:Solid_state_electronic_band_structure.svg
- 784