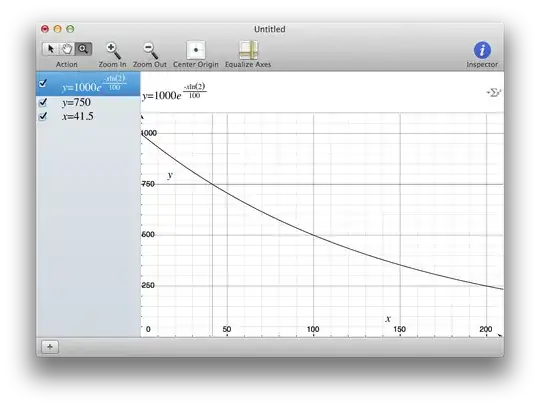
Radioactive element A radioactively decays into material B. If 75% of A and 25% of B are present, how many half-lives of material A have elapsed?
I was recently taught that the correct answer is "one half of a half-life has elapsed". However, due to the fact that the amount of radioactive material remaining scales exponentially (logarithmically) instead of in a linear fashion, wouldn't the answer be less than one half of a half-life?