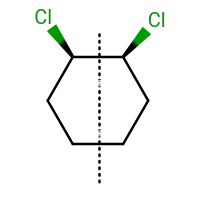
While passing a plane of symmetry through any compound inorganic or organic should it contain a minimum number of atoms or can it be passed without containing any atoms ?

While passing a plane of symmetry through any compound inorganic or organic should it contain a minimum number of atoms or can it be passed without containing any atoms ?

Yes , the plane of symmetry need not cross/pass through any atoms. The compound that you've drawn can show cis-trans isomerism. I believe you're talking about the cis form.