What is the drawn product drawn here the major product? I thought the radical (intermediate) is more stabilized at a primary carbon than at a tertiary one. 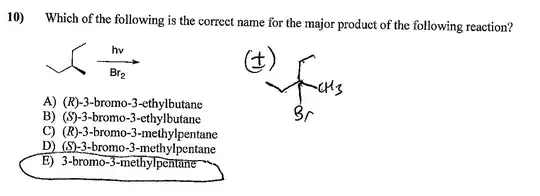
Asked
Active
Viewed 227 times
-3
Zhe
- 17,392
- 1
- 38
- 70
Sarah Smith
- 153
- 6
1 Answers
2
Carbon-centered radicals are electron deficient species in general--you have 7 electrons instead of the desired 8. This means that to first order, you can gauge the stability of a series of radicals the same way as you would for a series of carbocations. In this case, the tertiary cation and the tertiary radical would be the most stable in their respective series. So, no, definitely don't make a primary radical when you can make a tertiary radical.
Zhe
- 17,392
- 1
- 38
- 70
-
Ah thank you, that makes sense intuitively. I think I got confused because I was reading this website ( https://www.masterorganicchemistry.com/2013/04/12/a-fourth-alkene-addition-pattern-free-radical-addition/ ) on free radicals and in one of the examples, it says addition of a bromine radical has anti-markovnikov selectivity. Do you know why this might be? – Sarah Smith Feb 06 '18 at 21:42
-
The link you provide has to do with the addition of HBr in the presence of peroxides (Kharasch reaction), not the broomination of an alkane. – user55119 Jun 13 '19 at 18:04