I believe what is causing you confusion is the difference between optical activity and absolute configuration.
A molecule with a single stereocenter such as limonene and carvone have two stereoisomers, one rotates polarized light to the left (-) and the other to the right (+). Additionally, each one has its own absolute configuration in their chiral center, which can be R or S. The thing is, R and S configurations are not attached or are, anyhow, synonyms with (+) and (-) configurations. Carvone and limonene are good examples:

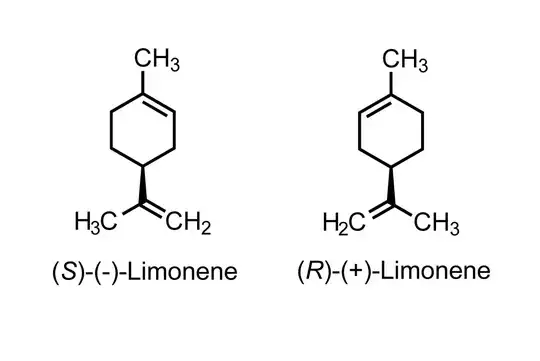
In carvone, the S-enantiomer rotates polarized light to the left and so it is named S-(+)-carvone. The S-enantiomer in limonene rotates polarized light to the right and so it is named S-(-)-limonene. The two nomenclature systems are different since they specify distinct properties.
I hope this answers your question. If not, I would be glad to help in anyway I can.

