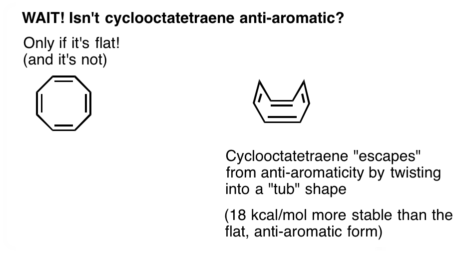
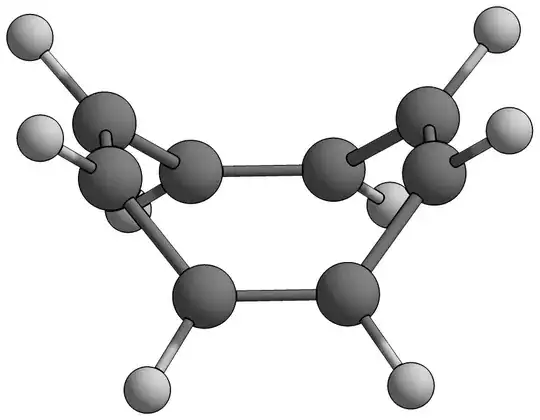
I understand that for cyclooctatetraene (COT) to "escape" the horrible prospect of becoming an anti-aromatic molecule, it must adopt a non-planar conformation.
The widely touted conformation of COT is said to be "tub" shaped. My sources (random links Google threw up) also concur on this particular "tub" conformation.
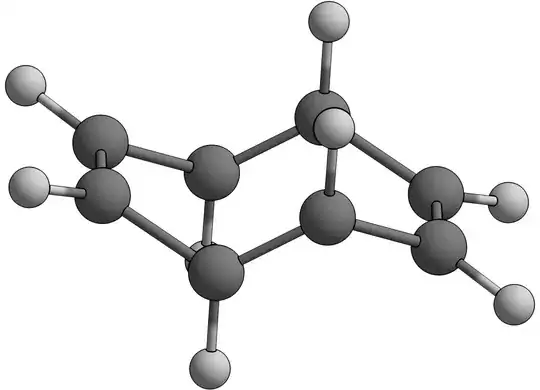
But why isn't it "chair" shaped instead?
Going along the same line of cyclohexane (non-aromatic), wouldn't a "chair" conformation reduce (even if it's a little bit) the steric repulsions due to crowding of hydrogens atoms on one side/face of the molecule?
Or even if "avoiding steric issues" isn't really the big idea here, then I could re-phrase my question as:
What stops COT from assuming a "chair" shaped structure?
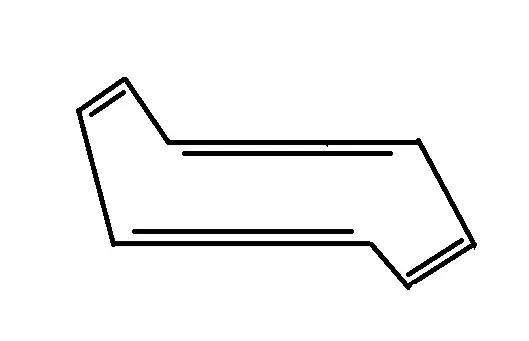
The "chair" structure that I have in mind for COT is something like this:
(I don't have access to a decent enough organic chemistry sketcher to have this drawn any better, sorry.)