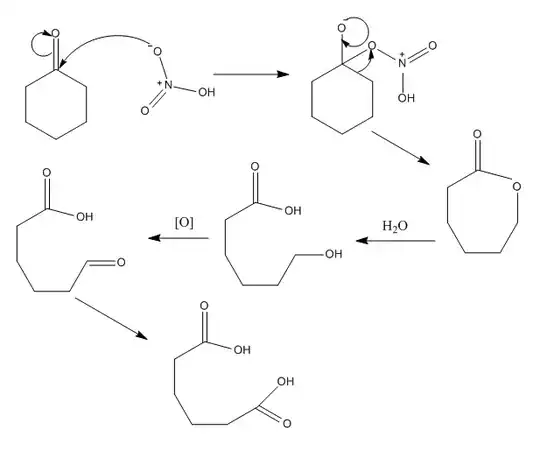
Can I get some help in figuring out the mechanism for this reaction.
Adipic acid is a molecule with two carboxylic groups,but I can't see how that happens. My initial thoughts involved that the carbonyl group in cyclohexanone is protonated,but I couldn't get any further as I couldn't figure out what would happen after that.

Asked
Active
Viewed 1.5k times
2
Daenys Targaryen
- 656
- 1
- 6
- 19
-
2Are you sure you want to use cyclohexanone $\ce{ C6H10O}$, not cyclohexanol $\ce{C6H11OH}$ for this reaction? This huge picture must carry a subliminal message, but I'm asking just to be 100% sure we are on the same page. – andselisk Jul 05 '17 at 11:10
-
I am sure I want to use cyclohexanone, but does it really matter because cyclohexanone can be converted into cyclohexanol. – Daenys Targaryen Jul 05 '17 at 12:05
-
1From what I remember at industrial scale cyclohexanone and cyclohexanol mixture is rectified, and cyclohexanone is used in the production of caprolactam, whereas cyclohexanol is utilized for the synthesis of adipic acid by oxidation. BTW, you need a catalyst ($\ce{NH4VO3}$) with 50% $\ce{HNO3}$ for that. – andselisk Jul 05 '17 at 12:12
-
https://chemistry.stackexchange.com/questions/4416/oxidation-of-ketones – Mithoron Jul 05 '17 at 15:25
1 Answers
1
Kushal Tripathi
- 149
- 8
-
1
-
1@Daenys Targarysn Further oxidation leads aldehyde to carboxylic acid. That's how the last step occurs. – Mockingbird Jul 05 '17 at 13:12
-
2That is incorrect, at very least mechanism of initial attack is written terribly. – Mithoron Jul 05 '17 at 15:11
-
@Mithoron Yes I am actually inclined to agree with you about the initial attack but its just what looked obvious to me .. – Kushal Tripathi Jul 06 '17 at 04:01
-
But the intermediate products formed while this transformation are correct so I just suggested this mechanism . – Kushal Tripathi Jul 06 '17 at 04:07
-