Note that this is not squaric acid as it has no double bond between the carbons holding the hydroxy groups.
-
1Too bad. Then it won't be stabilized in the anionic form the same way as squaric acid, and hence would be less acidic. – Ivan Neretin Mar 08 '17 at 14:26
-
@IvanNeretin but the alpha protons will still be labile ... in water I would imagine that there are 3 sp2 carbons the oxygens of which share one proton. It would be interesting to see a geometry optimised molecular model. – DarrenRhodes Mar 08 '17 at 14:29
-
What I want to know in particular is its pKa, 'cause I want to know whether it will form effervescence of CO2 with NaHCO3. – Aaron John Sabu Mar 08 '17 at 15:15
-
I should be able to get you an optimized geometry and I might be able to figure out a pKa. I'm practicing how to use Gaussian anyway so it should be useful to me to try it on a real example. – Tyberius Mar 09 '17 at 22:00
-
3The preferred IUPAC name for this compound is 3,4-dihydroxycyclobutane-1,2-dione. If you have any detailed questions about this name, you might want to consider asking a separate question (see also our guidelines on how to ask nomenclature questions). – Mar 10 '17 at 18:19
2 Answers
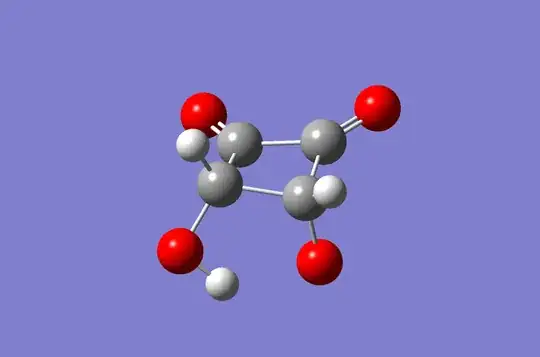
I know this isn't exactly an answer, but I think it might help in reaching a qualitative understanding. These are the geometry optimized structures of the protonated and singly deprotonated form of the molecule (I could not obtain a theoretical pKa; it is a much more involved calculation than I anticipated). I would expect the pKa to be fairly low, as the deprotonated structure would seem to be fairly stable due to hydrogen bonding.
(Images were obtained using GaussView. Optimized structures were from Gaussian Optimization calculation using APFD, Basis set 6-311G+(2d,p)).
- 11,686
- 10
- 42
- 86
-
You might also want to consider the trans-configuration of the molecule. – Martin - マーチン Mar 15 '17 at 04:44
-
@Martin That would probably help in the long to demonstrate if there is different behavior, but at this point I'm still stuck on getting a pKa value I can be confident in for this cis-configuration. – Tyberius Mar 15 '17 at 04:48
I guess if you change the structure of the molecule to get both the carbonyl grops opp to each other...(by Lobry De Bryn Van akelstein rearrangement) the hydroxyl carbon would be acidic enough to give a ppt with $\ce{NaHCO3}$
- 71,033
- 11
- 239
- 410
- 1,339
- 2
- 15
- 29
-
This is the sort of thing that I was thinking about in my comment above, " but the alpha protons will still be labile" etc ... – DarrenRhodes Mar 10 '17 at 22:15