Why does water dissociate to $\ce{H3O+ + OH-}$ instead of $\ce{H+ + OH-}$?
This question came to surface when I was learning about acids and bases, and learned this definition:
$\mathrm{pH=}-\log_{10}[\ce{H+}]$
Which after looking at Wikipedia, looks like a simplified definition, but it got me thinking - how can water have a pH of around seven if it dissociates into $\ce{H3O+}$ instead of $\ce{H+}$? Why does it do this instead of the expected dissociation?
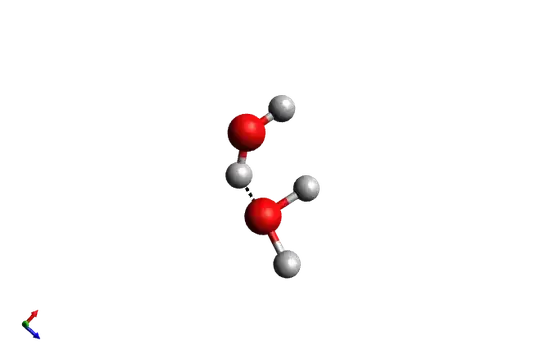 From there the electron from Hydrogen forms a lone pair with the oxygen of the same molecule forming $\ce{OH^-}$. Hydron ($\ce{H^+}$) is in fact a proton, a very reactive specie so is not so simple found it uncoupled and moreover the hydrogen is suppose to be bonded with an hydrogen bonding with the other water molecule so is more accurate use hydronium ($\ce{H_3O^+}$) instead of hydron...
From there the electron from Hydrogen forms a lone pair with the oxygen of the same molecule forming $\ce{OH^-}$. Hydron ($\ce{H^+}$) is in fact a proton, a very reactive specie so is not so simple found it uncoupled and moreover the hydrogen is suppose to be bonded with an hydrogen bonding with the other water molecule so is more accurate use hydronium ($\ce{H_3O^+}$) instead of hydron...