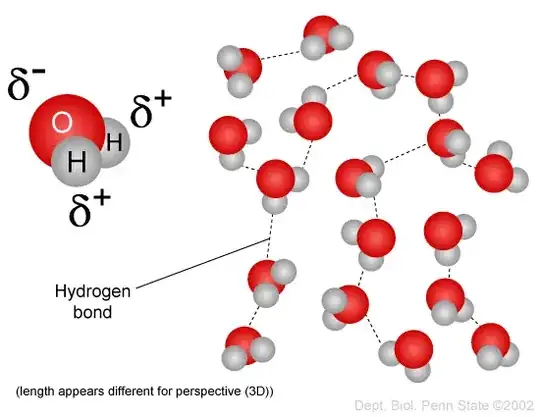
I learned that hydrogen bonding is formed from a 'sandwich' of hydrogen and either oxygen, nitrogen, or fluorine where nitrogen, fluorine, or oxygen is the bread.
Will oxygen gas and water form hydrogen bonds? The oxygen-hydrogen sandwich could be made, but oxygen gas has no hydrogen attached. Is it necessary for both molecules to contain hydrogen for hydrogen bonding to occur?
To make it clear, the question is: do both molecules have to contain hydrogen in order for hydrogen bonding to occur?