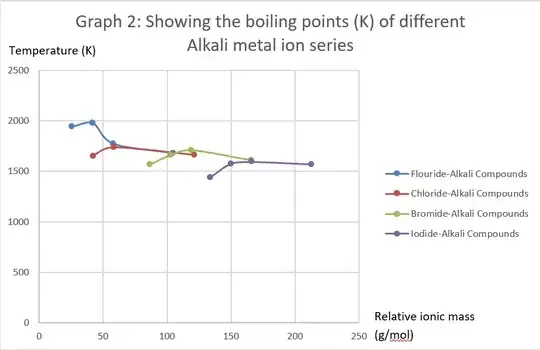
As you can see from the graph below, as we go down the blue fluoride-alkali metal series (alkali metal ion is varied from Lithium to Rubidium, which is represented by an increase in ionic mass on the graph), the boiling point increases then decreases. This is contrary to my expectation that the boiling point should continuously decrease due to an increase in ionic radius. The charge is obviously not a factor because it is consistent throughout. What could be the factor that causes this deviation?
Thanks!