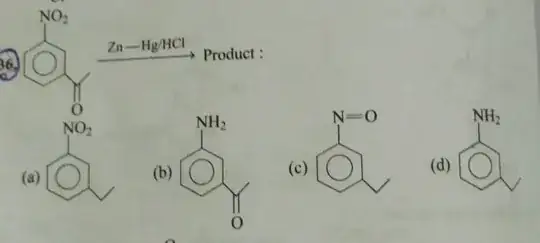
I would look at it in the following way: The oxidation state of the carbonyl carbon is +II, while the oxidation state of the nitrogen in the nitro group is +III. So nitrogen is in a higher oxidation state and it is also more electronegative. Thus, isn't it sensible that it gets reduced first before the carbonyl group? In fact, reduction of a nitro group is also possible using much milder reducing agents (e.g. only zinc dust).
In my experience, most questions of chemoselectivity can be answered by "thinking about it". However, if you would still like to have a good book for that, I would recommend Warren "Organic Synthesis: Strategy and Control" which contains a very nice treatment of chemoselectivity problems.