Why do alkanes with even number of carbon atoms have greater boiling points than those with odd number of carbon atoms?
-
3Because there is always a heavier alkane with equal number of carbon atoms than one with an odd number of carbon atoms. – aventurin Apr 23 '16 at 17:26
2 Answers
There is not really any difference between the boiling points of even and odd carbon alkanes. For single-chain alkanes the boiling point just goes up as a smooth curve versus chain length.
The melting points do show a small oscillating component with the even chains being higher. If we imagine the chains in their idealized zig-zag configuration, which would be relatively favorable for the solid state, we find that the even-length chains have a center of inversion which makes them fit better than odd-length ones. This effect fades, however, with increasing chain length.
Reference:
- 56,895
- 4
- 89
- 175
I think you asked your question poorly. Here is data from Wikipedia. Most of the BP's were given as ranges. I rounded some and some I took the average. But in all cases as the number of carbons increases, the BP increases.
alkane #Carbons B.P. (°C)
methane 1 −161.49
ethane 2 −88.5
propane 3 −42.2
n-butane 4 0
n-pentane 5 36
n-hexane 6 69
n-heptane 7 98
n-octane 8 125
nonane 9 151
decane 10 174
undecane 11 195
dodecane 12 216
I also fit the data to a 3rd order polynomial.
BP=-2.262586E+002 + 7.517034E+001*C - 5.350278E+000*C^2 + 1.810153E-001*C^3
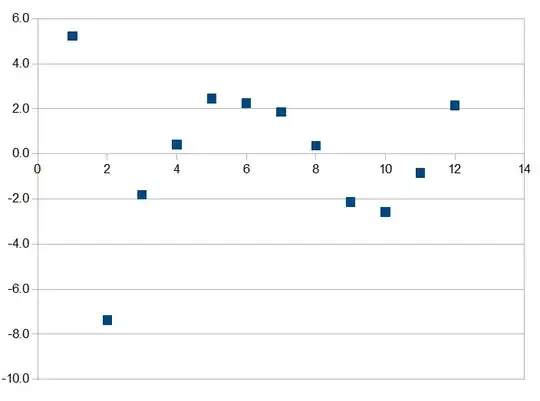
alkane #Carbon BP(data) BP(fit) error
methane 1 -161.5 -156.3 5.2
ethane 2 -88.5 -95.9 -7.4
propane 3 -42.2 -44.0 -1.8
n-butane 4 0.0 0.4 0.4
n-pentane 5 36.0 38.5 2.5
n-hexane 6 69.0 71.3 2.3
n-heptane 7 98.0 99.9 1.9
n-octane 8 125.0 125.4 0.4
nonane 9 151.0 148.9 -2.1
decane 10 174.0 171.4 -2.6
undecane 11 195.0 194.2 -0.8
dodecane 12 216.0 218.1 2.1
Here is a plot of the residuals. As Oscar Lanzi indicated there is no evidence of an even-odd trend.
- 22,242
- 2
- 36
- 80