I was wondering whether allenes can form rings; geometrically speaking, this seems like it would be energetically unfavorable due to their 180 degree linear geometry.
Asked
Active
Viewed 518 times
7
2 Answers
7
Yes they do. Such compounds are even found in nature, e.g. vernonallenolides.
aventurin
- 7,200
- 3
- 27
- 38
-
I think OP means "Do allenes react with themselves to form rings," not "Do allenes exist within rings?" I like the post, I just don't think it answers the question's intended meaning. Actually, I may well be wrong. Maybe OP could elaborate. – SendersReagent Mar 09 '16 at 23:01
-
Well, I guess my main question was whether rings of allenes would be stable enough to exist at all, which was answered by the examples above...However, your question is an interesting one too that I would love to know the answer to. – John Smith Mar 10 '16 at 23:30
6
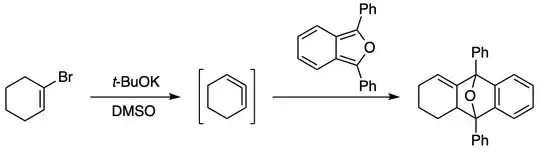
As small as six-membered rings with allenes can exist, albeit fleetingly. Cyclohexa-1,2-diene has been known since a 1966 report by Georg Wittig (Angew. Chem. Int. Ed. 1966, 5 (9), 846), in which it is prepared by direct elimination of HBr from a vinyl bromide using t-BuOK:
For a more modern take on the matter, see e.g. Nat. Chem. 2018, 10 (9), 953–960 where Neil Garg and coworkers report the generation of azacyclic six-membered cycloallenes. The allene is generated by treatment of a 1,2-silyl triflate with CsF (this strategy will be familiar from benzyne chemistry). The cycloallene intermediates are trapped in various cycloaddition reactions.
orthocresol
- 71,033
- 11
- 239
- 410
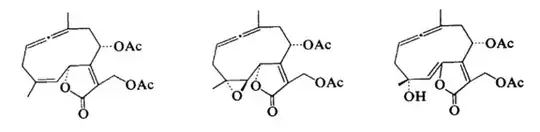

That being said, these kinds of systems are not common.
– Lighthart Mar 07 '16 at 21:57