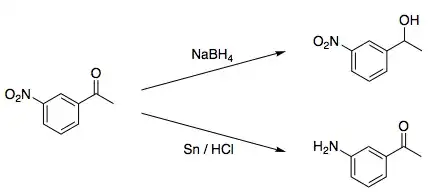
The key question is how the reduction by the two reagents proceeds.
For tetraborohydride the reduction proceeds as a standard nucleophilic addition of hydride ion to carbonyl group.
For tin/HCl the reduction proceeds as a series of one-electron transfers from metal surface mixed with protonation. It seems that the lowest free orbital in the compound belongs mostly to nitro group (which makes sense as it has a strong π-withdrawing effect, stronger than carbonyl group), so it is reduced to a nitroso group and then further via a hydroxylamine to an amine. It is possible to stop the reduction at intermediate stages under some conditions.
So the question is, why borohydride does not reduce the nitro group. It should be able to do so, as a strong reducing agent which is able to reduce iron from solutions. A possible answer is that extremely small size of nitro group with a net negative charge on the nitro group prevents formation of a stable adduct with hydride ion.