If we talk about a series of molecules: ethene, prop-1-ene, but-2-ene, etc., we see both hyperconjugation (which increases stability) and steric hindrance (which decreases stability). So which one is more powerful i.e. which of these molecules will be most stable?
-
2Stable with respect to what? – NotEvans. Jan 01 '16 at 15:21
-
1@NotNicolaou, probably thermodynamic stability with respect to each other. In which case ethene would be the most stable because of most no. of alpha-H and least steric hindrance. – Tamoghna Chowdhury Jan 01 '16 at 15:23
-
@NotNicolaou As in my textbook, more number of alkyl groups attached to double-bonded carbon cause increase in steric hindrance which make the molecule unstable (simply unstable to exist). – another 'Homo sapien' Jan 01 '16 at 15:27
-
Can you cite the textbook so I can read the whole thing? – NotEvans. Jan 01 '16 at 15:27
-
1@Tamoghna my teacher told that more number of alkyl groups would provide more alpha-H and thus increase hyperconjugation and stability, though i doubt whether those hydrogens will actually be alpha-H. – another 'Homo sapien' Jan 01 '16 at 15:30
-
1@NotNicolaou actually this one just came to my mind and i discussed it with my teacher, but was not satisfied with the answer. The point that "more number of alkyl groups attached to double-bonded carbon cause increase in steric hindrance which make the molecule unstable" was told by my teacher. – another 'Homo sapien' Jan 01 '16 at 15:32
-
You're right, those will become beta-H and won't contribute to the stability. – Tamoghna Chowdhury Jan 01 '16 at 15:35
-
1It's not just double-bonded C, but rather any C atom attached to a functional group. – Tamoghna Chowdhury Jan 01 '16 at 15:36
-
So, simply, would more alkyl groups increase stability? (i didn't get what you just posted) – another 'Homo sapien' Jan 01 '16 at 15:41
1 Answers
It's a contradict to explain the stability of substituted alkenes by using hyperconjugation and steric hinderance both. I think, it is more better to consider heat of hydrogenation factor in case for stability of substituted alkenes. The stability of substituted alkenes is explained by Hyperconjugation factor while the stability of geometrical alkenes (cis-trans) is explained by steric hinderance.
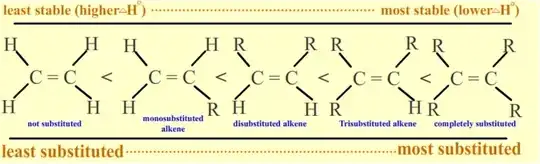
The stability of alkene can be determined by measuring the amount of energy associated with the hydrogenation of the molecule. Since the double bond is breaking in this reaction, the energy released in hydrogenation is proportional to the energy in the double bond of the molecule. This is a useful tool because heats of hydrogenation can be measured very accurately. The ΔHo is usually around -30 kcal/mol for alkenes. Stability is simply a measure of energy. Lower energy molecules are more stable than higher energy molecules. More substituted alkenes are more stable than less substituted ones due to hyperconjugation. They have a lower heat of hydrogenation. The following illustrates stability of alkenes with various substituents:
In disubstituted alkenes, trans isomers are more stable than cis isomers due to less steric hindrance because in trans bulky groups are in opposite sides so less repulsion between them as there is more repulsion in case of cis due same side of bulky groups. Also, internal alkenes are more stable than terminal ones. See the following isomers of butene:
Overall stability : trans-but-2-ene > cis-but-2-ene > propene > ethene
- 969
- 1
- 5
- 14
-
I still have a doubt. In your first diagram, if we look at the third molecule (let it be the trans isomer) and fifth molecule, how will you explain the stability as fifth molecule has more steric hindrance than third one. I cannot understand "The stability of substituted alkenes is explained by Hyperconjugation factor while the stability of geometrical alkenes (cis-trans) is explained by steric hinderance". – another 'Homo sapien' Jan 01 '16 at 16:16
-
It is simple. here is a trick. when u compare all substituted alkene which have different molar mass with each other (not for geometric isomers i.e. cis-trans), take hyperconjugation factor. In case of alkenes of same molar mass i.e. with geometrical isomers, take steric hinderance factor. – solanki... Jan 01 '16 at 16:31
-
So, let your 'R' in third and fifth molecule have same molar mass. Now you can apply both steric hindrance and hyperconjugation because they are not geometrical isomers. So which one is more stable? – another 'Homo sapien' Jan 01 '16 at 16:35
-
Actually my question is that which one of hyperconjugation and steric hindrance is more effective in increasing stability of the molecule? – another 'Homo sapien' Jan 01 '16 at 16:36
-
Trans alkenes are only more stable than cis alkenes in the case of acyclic systems. Trans-cycloalkenes smaller than about 12 carbons are unstable due to ring strain. See here for details. – bon Jan 01 '16 at 17:50
-
@kiransolanki I get your point now of not comparing compounds of different molar masses by steric hindrance. Then why did my teacher tell me hex-3-ene, pent-2-ene and but-2-ene for comparing steric hindrance? (This is what made me confuse) – another 'Homo sapien' Jan 02 '16 at 04:56
-
@aayushsrivastav. don't know. what is going on ur teacher mind that time. but, here all three hex-3-ene, pent-2-ene and but-2-ene can be compare by hyperconjugation. – solanki... Jan 02 '16 at 04:59