Here's an image:
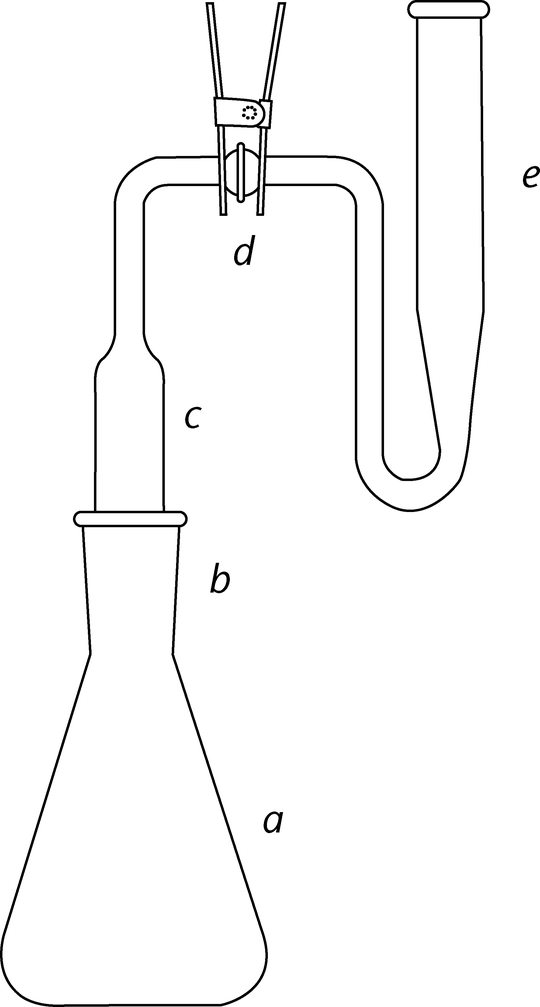
The left side is designed to be fitted into a beaker, and it captures gas evolved during a reaction. The right side can be filled with a liquid to then react with that gas. We use it to capture ammonia from a decomposition reaction, with an indicator in the right half as part of an identification test. I'm sure it must have a name, it's a commercial piece of glassware, but the box we keep it in in the lab doesn't identify it.