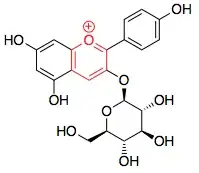
I am trying to figure out which rings in callistephin are aromatic:
I know that the benzene rings definitely are aromatic, but I'm not sure about the ring with the positively charged oxygen (highlighted in red).
How does the Hückel $4n+2$ rule apply to this ring?