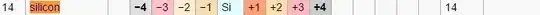According to List of oxidation states of the elements, silicon has a possible oxidation state of $-4$.
Now, I've been looking everywhere for a compound that contains Si(-IV), but I cannot find any definitive references. Perhaps one of the synthetic carbonyls of silicon has this OS? I've seen references to $\ce{[Mo(CO)_4]^{-4}}$ and $\ce{[W(CO)_4]^{-4}}$ in my research, but silicon never shows up.
I am also uncertain as to how these carbonyls come together, and whether or not they actually complex in the same way for metalloids as for the transition metals.