In addition to @Mithoron's comments and the Wikipedia he linked:
This is an important consideration in photovoltaics, as silicon forms the basis of the internal structure of solar technology (and image sensors). According to the PVEducation.org page Semiconductor Structure,
Each silicon atom has four valence electrons which are shared, forming covalent bonds with the four surrounding Si atoms.
Diagrammatically, the website includes the following schematic:
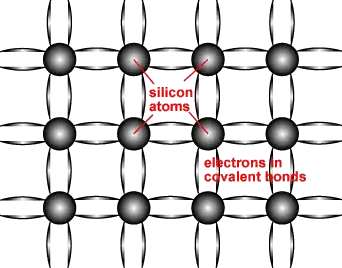
The caption reads:
Schematic representation of covalent bonds in a silicon crystal lattice. Each line connecting the atoms represents an electron being shared between the two. Two electrons being shared are what form the covalent bond.
In terms of germanium, according to the WebElements page Group 14 Elements,
Tin and lead are very metallic although one modification of tin known as grey tin has the same diamond structure [as carbon] as does germanium and silicon.
