Looking at the examples in Tautomer on Wikipedia, it seems that the hydrogen needs to travel a significant distance (~20Å) for the interconversion to take place. What lets it happen so fast? Why interconversion is preferred over maintaining its position?
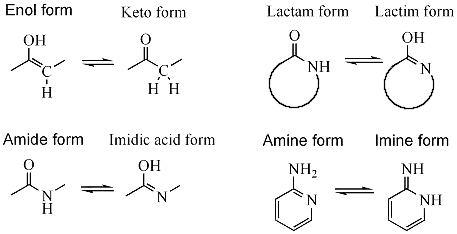 (source)
(source)