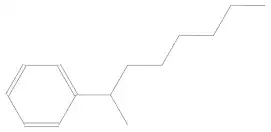
What is this compound's IUPAC name?
1) 2-phenyloctane.
2) octan-2-ylbenzene.
Is using phenyl correct or is taking benzene as the base correct?

What is this compound's IUPAC name?
1) 2-phenyloctane.
2) octan-2-ylbenzene.
Is using phenyl correct or is taking benzene as the base correct?