There are two main types of glucose, $\alpha$-D-(+)-glucose and $\beta$-D-(+)-glucose.
Can anyone please explain what is $\alpha$,D or (+)?
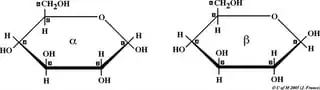
So (maybe by permutation and combination), how many types of glucose are there?
- 44,013
- 13
- 159
- 319
- 1,673
- 5
- 20
- 29
-
A highly related question: http://chemistry.stackexchange.com/questions/7308/whats-the-difference-between-alpha-glucose-and-beta-glucose – Curt F. Feb 19 '15 at 06:02
-
@CurtF. It would be better to consult meta before introducing a new tag to a whole lot of questions. I personally did not see any benefit in the new tag. The community might disagree with me on this, so please go ahead and post to meta about it. – Martin - マーチン Feb 19 '15 at 07:13
-
Sorry I did not realize the protocol. I probably won't write a post on meta because I did not maintain my own separate records of the posts tagged, and without those posts to display, I can't make a good argument for the tag. I don't feel like re-searching through old questions to find the ones I already found. I guess we won't have a "carbohydrates" tag here after all, at least not any time soon. – Curt F. Feb 19 '15 at 07:18
1 Answers
Glucose can exist in the open form or cyclic form.

In the open form, there are only two types, D-glucose (illustrated in Fischer projection above) and L-glucose which is the mirror image of the above.
Experimentally, D-glucose rotates plane polarize light clockwise, hence the symbol "+", and L-glucose rotates plane polarize light counterclockwise, hence the minus symbol. For more information on the meaning of + versus - see: http://en.wikipedia.org/wiki/Dextrorotation_and_levorotation
In the cyclic form, there is an additional chiral center, so there is alpha (where 3 OH groups are on the same side of the ring) vs beta (where 2 OH groups are on each side of the ring) as ilustrated in the drawing of the question.
Each of alpha and beta have a D and an L form, so there are 4 types in the cyclic form.
So in summary, there are only two types in the open form, but 4 types in the cyclic form.
- 40,570
- 2
- 85
- 181
-
1So if "D" and "(+)" have the same meaning why do we have to write both of them? Or is there something like "D-(-)" ? – NeilRoy Feb 18 '15 at 15:04
-
4No, it just happens to be that way in this case. For glucose, D experimentally is + and L experimentally is -, but for other molecules it can be reversed. Read more about this here: http://en.wikipedia.org/wiki/Chirality_(chemistry) – DavePhD Feb 18 '15 at 15:14
-
It's worth noting that in aqueous solution the forms interconvert fairly rapidly. So for D-glucose, or D-(+)-glucose if you prefer, three species are interconverting: the open chain D-glucose, the cyclic $\beta$-D-glucopyranose, and the cyclic $\alpha$-D-glucopyranose. (Glucopyranose is a name that expicitly indicates the six-membered ring closed form of glucose.) – Curt F. Feb 18 '15 at 19:21