I have performed an experiment where I had some test tubes with the reagents and different amounts of catalyst. I started the reactions and measured the time they took.
Then I calculated the rate of reaction and plotted it in function of the amount of catalyst (in mL) in each test tube.
However I can't decide between a logarithmic fit line and a linear one (though the logarithmic one has a higher $R^{2}$). The data points are below.
If it helps, the reaction we used was the one of potassium permanganate with oxalic acid. We also added sulfuric acid and the catalyst used was manganese sulphate.
What would make more sense?
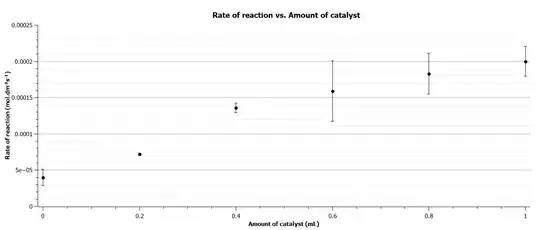
0 4e-05
0.2 7.22e-05
0.4 0.000136
0.6 0.000159
0.8 0.000183
1 0.0002