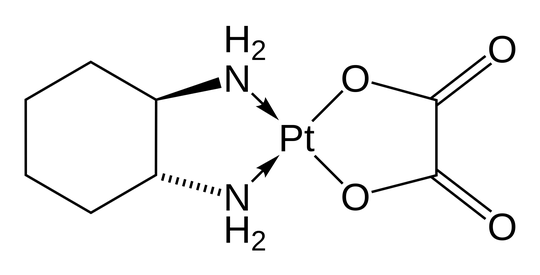
What do the arrows mean in the Structural Formula of Oxaliplatin? I haven't seen these and couldn't find anything explaining them.
Asked
Active
Viewed 53 times
1 Answers
3
The free diamine has a nonbonding pair on each nitrogen atom; the arrows indicate that these pairs are incorporated into dative bonds to form the complex.
Thus the complexed diamine has two positive formal charges with the compensating negative formal charges on the platinum. Note, however, that the oxidation state of the platinum, which is calculated assuming ionic rather than covalent bonding, is +2 (which is commonplace in platinum complexes).
Oscar Lanzi
- 56,895
- 4
- 89
- 175
-
Why would the nitrogens even react with the platinum? Isn't their valence full with the C and 2 H's, via the octet rule? – imrobert Feb 21 '24 at 03:42
-
1@imrobert The nitrogen atoms in the amine carry a non-bonded pair of electrons; hence, they are Lewis bases. The platinum(II) cation on the other hand has a gap, "is missing" electrons to complete its outer valence shell; hence, it is a Lewis acid. In consequence, the Lewis base donates electrons the Lewis acid accepts. (There is some share of electron density between the two to establish a chemical bond though.) – Buttonwood Feb 21 '24 at 10:30