Factors influencing the acid strength of carboxylic acids
For starters, it should have been considered that the acid strength of carboxylic acids depends on
electron releasing groups (which weaken the acid) / electron withdrawing groups (which strengthen the acid).
Number of such groups (higher number of ERGs will lead to weaker acids, while higher number of EWGs will lead to stronger acids)
The distance of the group from the carboxyl group (an EWG kept closer will lead to a stronger acid; an ERG kept further away will lead to a stronger acid.)
Inductive effect, due to the $\ce{-\color{red}{F}}$ group, is distance dependent, which means that the effect will become weaker as the group is placed further away. Check out the below example:

Apply a similar logic to the situation.
The reduction in $\mathrm{\color{red}{-I}}$ effect of fluorine is much more and because of that the formic acid turns out to be stronger.
Also, 3-fluoropropanoic acid has one EWG, but some ERGs before it [$\ce{\color{navy}{F}-\color{blue}{C2H4}-\color{magenta}{COOH}}$], and the alkyl group in between has some electron releasing tendency. There is competition between those two effects and $\ce{-\color{red}{F}}$ being further away from the COOH group leaving a $\mathrm{\color{green}{+I}}$ group in between acts as the differentiating factor.
Thus the order turns out in this fashion.
I would suggest you to read these paragraphs from our Chemistry reader:

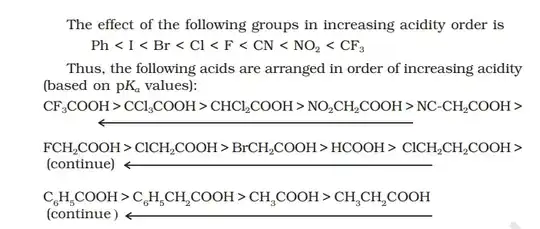
Image sources:
https://shorturl.at/qzFSY (a Google image)
https://ncert.nic.in/textbook.php?lech2=3-5
This answer is derived from my learnings from NCERT, Class XII, Chemistry Volume II.




I will upload a snapshot from my chemistry textbook that gives the complete order of all carboxylic acids. Read whatever factors I have said, understand those factors completely and then have a look at the order.
– Harikrishnan M Dec 26 '23 at 01:20