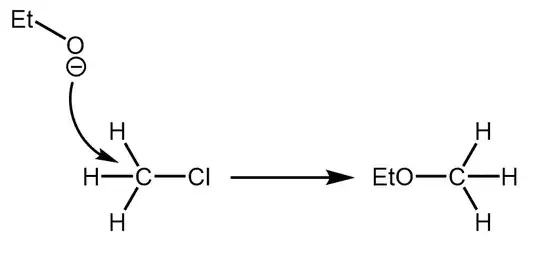
Consider the reaction of chlorocyclohexane with ethoxide in a suitable inert solvent. The major product according to my textbook is cyclohexene, which implies E2 reaction pathway. Why not SN2?
$\ce{EtO^-}$ is an unstable anion, so why can it act as nucleophile rather than acting as a base?