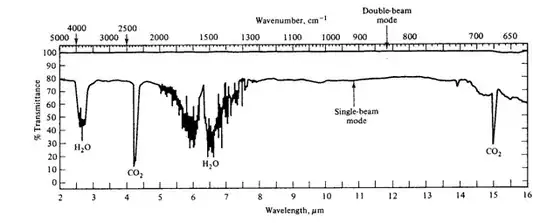
In the IR of air shown below, the region for water at 1500 cm-1 is hard to individually assign peaks due to their "spikey" nature. Is there any specific reason for this or does it just happen? Is it potentially due to interactions from other hydrogen in the air sample maybe binding to the water or interfering with it in some way?
Asked
Active
Viewed 232 times
3
-
3This may help: https://chemistry.stackexchange.com/a/116536/79678. – Ed V Mar 22 '23 at 12:38
-
2Instead of spikes, the proper spectroscopic term is rotational line structure. Those are actual transitions. – AChem Mar 22 '23 at 14:03
-
Can the rotational line structures ever be individually identified to a vibrational mode or are there too many that it's not possible? – Audrix Mar 22 '23 at 14:11
-
2yes. A standard undergraduate experiment is to measure at the vibrational -rotational spectrum of HCL. In the HCL as in the water the spectrum measures transitions from rotational levels associated with one vibrational level to rotational transitions in the next vibrational level. (A spectrometer with good resolution is needed also, $1, \mathrm{cm}^{-1}$ or better) – porphyrin Mar 23 '23 at 12:13
1 Answers
1
Those "spikes" are actually the rotational transitions of water. With a high enough resolution spectrometer, you can see the rotational structure associated with a given vibrational energy level for most gas phase molecules.
Tkh97
- 367
- 2
- 8
-
Since the gas molecules are separated the lines are not collisionally broadened as theynare in the liquid spectrum – jimchmst Jul 11 '23 at 07:27
-
@jimchmst You'll still see collisional broadening in the gas phase at pressures greater than 1 Torr. You can start to assume doppler broadening at low pressures, but in most cases, collisions still affect the line shape – Tkh97 Jul 11 '23 at 15:47