I read about a hypothetical compound called hexazine on Wikenigma. It's a 6-membered ring all of whose atoms are nitrogen and they form alternate single and double bonds just similar to benzene. But it also says that this would be highly unstable. How can it be so much unstable when it's structure resembles so much to that of benzene which is exceptionally stable. Has it something to do with nitrogen's compact size?
-
2Related: https://chemistry.stackexchange.com/questions/53864/ring-compounds-of-non-carbon-atoms – Nilay Ghosh Mar 04 '23 at 04:19
-
4The decomposition to 3 molecules of nitrogen gas would be highly exothermic – Waylander Mar 04 '23 at 07:06
-
2Well, chemistry is like that: all elements are different, even if they are neighbors. Size is certainly not an explanation: one N to another N is the same as one C to another C. – Ivan Neretin Mar 04 '23 at 07:21
-
1In addition you might ask what happens when you place 6 N atoms in bonding proximity in a vaccuum, that is, what is (or are) the most stable structures of N molecules containing 6 atoms. – Buck Thorn Mar 04 '23 at 07:48
-
4It's hard to beat that NN triple bond. You can make N clusters but they are not rings: https://arxiv.org/ftp/arxiv/papers/1805/1805.00835.pdf – Buck Thorn Mar 04 '23 at 07:51
-
1But why does benzene not decompose spontaneously into three acetylene molecules ? The reverse equation is possible for acetylene being transformed into benzene. Why is $\ce{N_2}$ never transformed into hexazine ? – Maurice Mar 04 '23 at 11:14
-
Related: "Azidoazide azide" at acs.org or Wikipedia – Poutnik Mar 04 '23 at 13:17
-
Should't hexazine be aromatic? May be that could provide some stability. – Proscionexium Mar 04 '23 at 13:20
-
3Notice relative bonding energies in simple, double and triple bonds of carbon and nitrogen. Get the benzene stabilization energy due aromaticity. Evaluate and conclude. – Poutnik Mar 04 '23 at 17:09
2 Answers
How can it be so much unstable when it's structure resembles so much to that of benzene which is exceptionally stable.
Benzene is not exceptionally stable to start with. As you substitute more and more CH groups with N in benzene, the enthalpy of formation (already positive for benzene) becomes larger and larger (e.g. 1,3,5 triazene). A positive enthalpy of formation tells you how much energy is released when the molecule turns back into elements. (It does not tell you about the activation energy needed for the process, so a molecule can exist even if a positive enthalpy of formation).
Playing around with the different unsubstituted species on MolCalc, it seems that the predicted enthapies of formation are higher when two nitrogen atoms are directly next to each other. In hexazine, there would be 6 of those pairs.
There are no examples of pentazine or hexazine. There are different tetrazines, but 1,2,3,4 tetrazine only exists fused to benzene (in the picture below, compounds (1), (4), and (5) have not been observed yet).
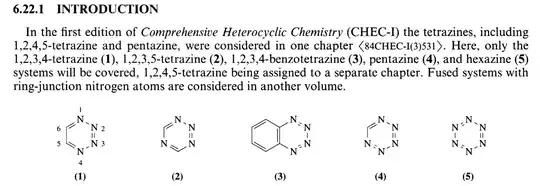 Source: https://www.sciencedirect.com/science/article/pii/B9780080965185001386
Source: https://www.sciencedirect.com/science/article/pii/B9780080965185001386
Like the comments of the question already say, bonding in elemental carbon is very different from that in elemental nitrogen. Similarly, substituting N for CH in ethane yields hydrazine, a rocket fuel. So while the Lewis structure look the same, many properties change dramatically when you substitute N for CH.
- 40,170
- 8
- 67
- 183
-
1Hydrazine isn't explosive... unless you mix it with oxidant, but so is ethane. There are plenty of nitrogen rich explosives, though, like tetrazene explosive – Mithoron May 01 '23 at 15:57
-
1@Mithoron Point well taken, I removed that bit. Ethane has a negative enthalpy of formation, while hydrazine has a positive enthalpy of formation. Thus, the latter can be used as a monopropellant in thrusters operating in outer space, e.g. here. – Karsten May 01 '23 at 21:33
An aromatic five-nitrogen ring is known as the pentazolate ion, $\ce{N_5^-}$. Among several documentations of this anion, which is isoelectronic with the cyclopentadienyl anion, the Wikipedia article identifies an isolated salt with moderately good thermal stability:
In 2017, white cubic crystals of the pentazolate salt, $\ce{(N5)6(H3O)3(NH4)4Cl}$ [the article and cited reference render the anion first in the formula] were announced. In this salt, the $\ce{N_5^-}$ rings are planar. The bond lengths in the ring are 1.309 Å, 1.310 Å, 1.310 Å, 1.324 Å, and 1.324 Å.[1] When heated, the salt is stable up to 117 °C, and over this temperature it decomposes to ammonium azide.[1]
Crystal packing presumably stabilizes the anion in the presence of protic species that would surely react with it in solution.
Cited Reference
- Zhang, Chong; Sun, Chengguo; Hu, Bingcheng; Yu, Chuanming; Lu, Ming (26 January 2017). "Synthesis and characterization of the pentazolate anion cyclo-N5ˉ in (N5)6(H3O)3(NH4)4Cl". Science. 355 (6323): 374–376. Bibcode:2017Sci...355..374Z. doi:10.1126/science.aah3840. PMID 28126812. S2CID 206651670.
- 56,895
- 4
- 89
- 175
-
1Cool reference! From the editorial introduction: "The flip side of the robust stability of N2 is the instability of any larger molecules composed exclusively of nitrogen. These molecules nonetheless remain enticing targets for explosive and propellant applications." – Karsten Mar 04 '23 at 20:05
-
$\ce{N3- and N5+}$ also exist, see here. They aren't aromatic, though (not even a ring). – Karsten Mar 04 '23 at 20:09
-
@karsten the WP article says chemists trued to synthesize $\ce{N5^+N5^-}$ for use as rocket fuel. That apparently failed, but we do have $\ce{LiN5}$ synthesized wt high pressure. Might that work with an appropriate pentazenium compound? – Oscar Lanzi Mar 04 '23 at 20:11
-
1