I have a doubt about the correlation between eutectic plots and freezing point lowering. The freezing point lowering, as well as all colligative properties, is defined in the presence of a volatile solvent and a non volatile solute. In fact, by adding NaCl to water, a freezing point lowering of the resulting solution is obtained, which follows this trend:
However, a similar trend is also obtained by adding ethanol (solute) to water:
But ethanol is volatile, so it shouldn't be referring to freezing point lowering.
So, is it correct to talk about freezing point lowering in the context of these diagrams? Or do we speak about freezing point lowering only in reference to the phase diagram of a single substance, like this?
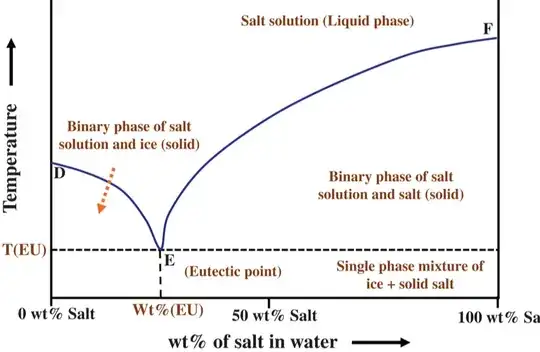
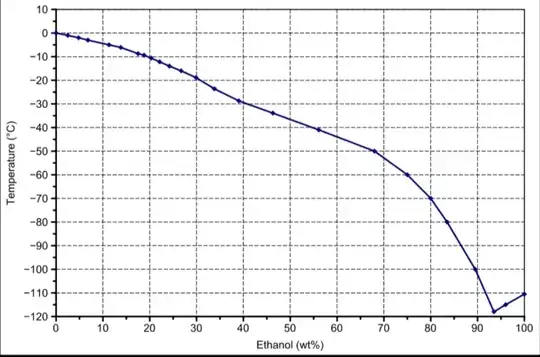
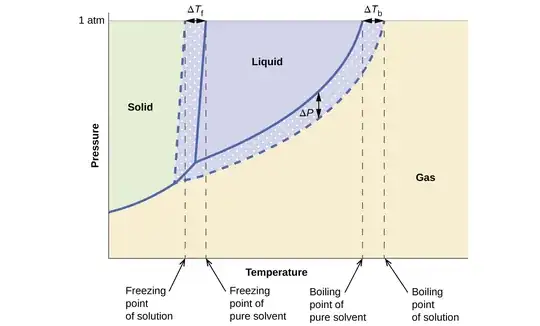
Therefore, in the first plot, that trend is given by the colligative property (freezing point lowering) because we have a non-volatile solute. Does the second graph always refer to freezing point lowering even if the solute is volatile?
– Luckenberg Feb 17 '23 at 10:44