While I understand why aromatic aldehydes and ketones do not give positive Fehling's test, I do not know why Glyoxal and Glyoxylic Acid give a negative test too. Nothing has been written about these two compounds in my study material and I was hoping someone could help me out with the reasoning. I figure it's something to do with presence of alpha-H but I'm not sure.
Asked
Active
Viewed 825 times
1
-
See: https://chemistry.stackexchange.com/questions/90778/does-acrolein-give-fehlings-test/136835 – Nilay Ghosh Jun 13 '22 at 02:23
-
1I have seen that question, and the answer did not satisfy the user who asked nor did it answer my question. – Dhruv Kaushik Jun 13 '22 at 04:10
1 Answers
4
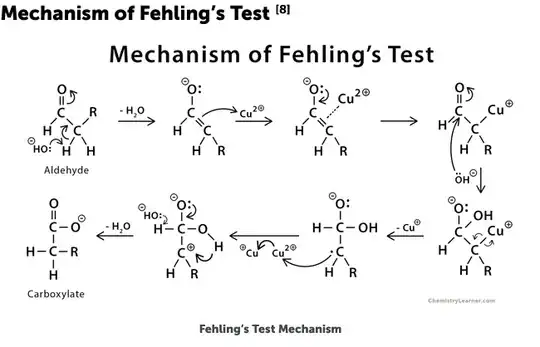
There is a good diagram of the mechanism here that I have reproduced below.
The first step is the formation of an enolate anion of the aldehyde by removal of the alpha proton by hydroxide. The enolate then binds to Cu2+. If the enolate cannot be formed then the reaction cannot proceed. Glyoxal and Glyoxylic Acid cannot form enolates so they give a negative test. The only proton that can be removed from glyoxal to form an enolate is the proton on a -CHO group. They are far less acidic than the alpha protons of an aldehyde. Glyoxalic acid obviously deprotonates at the carboxylic acid group, so no enolate forms.
Waylander
- 22,035
- 4
- 37
- 58