CONTEXT:
Here's reaction of alkyl halide with aqueous $\ce{KOH}$
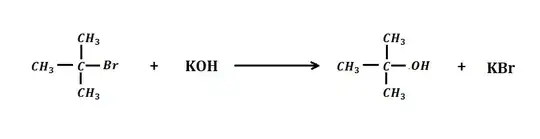
and here's the reaction for dehydrohalogenation by alcoholic $\ce{KOH}$

Question
Initially $\ce{KOH}$ is aqueous. On reacting with alkyl halide, it produces alcohol product. Does the alcohol not make the initially aqueous $\ce{KOH}$ alcoholic as a result producing alkene in the later part of the reaction? If so, wouldn't the reaction initially produce alcohol and produce alkene from the remaining reactants?