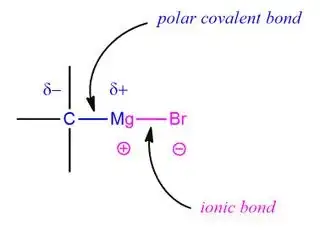
I just want to confirm if the $\ce{R-Mg}$ bond is a covalent bond which undergoes heterolytic fission during reactions while the bond between magnesium and halogen atom is ionic. Am I right or wrong?
Asked
Active
Viewed 1,710 times
3
-
3The R-magnesium bond in a Grignard reagent is polar covalent with carbon being the negative end of the dipole, which explains its nucleophilicity. And the magnesium-halogen bond is largely ionic. – R_Squared Sep 12 '21 at 03:06
-
1https://chemistry.stackexchange.com/questions/429/why-do-magnesium-and-lithium-form-covalent-organometallic-compounds – Mithoron Sep 13 '21 at 23:20
1 Answers
5
Yes, you are correct. The carbon-magnesium bond in a Grignard reagent is polar covalent with carbon being the negative end of the dipole, which explains its nucleophilicity and the magnesium-halogen bond is largely ionic.
Nilay Ghosh
- 25,509
- 26
- 91
- 197