according to wikipedia :
Tollens' reagent (chemical formula Ag(NH3)2OH) is a chemical reagent used to distinguish between aldehydes and ketone functional groups along with some alpha-hydroxy ketones which can tautomerize into aldehydes
you could handle this problem considering the first step in tollen's test which is a redox reaction :

For tollen's test to give positive, your substrate must have a relatively high oxidation potential, that's why aldehydes give positive while ketones don't:
- aldehydes can be oxidized easier than ketones as hydrogen of
-aldehydes is more electron-donating than ketones' alkyl group this causes the electrons occuping the MO of carbonyl oxygen to be higher in energy and thus more unstable and relatively easier to get lost (i,e better HOMO-LOMO interaction)
- alkyl shift (in ketones) is much harder than hydrogen shift (in aldehydes)
- alkyl groups in ketones stabilize electrons in carbonyl group making it harder to break that bond
Keeping that in mind, let's analyze alpha hydroxy ketones:
hydroxyl group would decrease the dipole effect of carbonyl oxygen and stabilize carbonyl electrons (i,e, decrease its oxidation capacity)
If alpha hydroxy ketone had to be deprotonated first before being oxidized, this step produces conjugated structure which can stabilize carbonyl group, this decreases the tendency to being oxidized. Evidence to this is that aromatic aldehydes don't react with Fehling's reagent (which is a weaker oxidizing agent than Tollen's) due to conjugation and increased stability of carbonyl group. If Tollen's reagent already can't reduce ketones how would it reduce stabilized ketones?

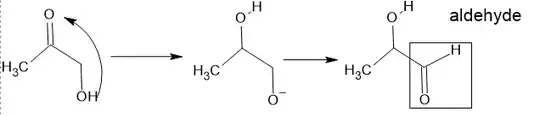
the only way alpha-hydroxy ketones can react with Tollen's reagent is as wikipedia states through tautomerization. this necessitates that hydroxyl group of the ketone be a terminal one (i,e primary hydroxyl group), there are some exceptions such as benzoin
Benzoin
Benzoin is oxidized using copper(II) acetate in catalytic amount (1), so this means that it would be easier for silver ion to oxidize it. Another important aspect is that Fehling's reagent (Copper-based oxidizing agent) doesn't oxidize aromatic aldehydes besides the fact that benzoin can't tautomerize into an aldehyde. My explanation is that
oxygen of carbonyl groups are cross-conjugated and not fully conjugated as the main conjugated path involves the two phenyl rings and the two carbons P-orbitals. So, I think it will be easily deprotonated and oxidized

references:
(1) Marvin Weiss and Mildred Appel
Journal of the American Chemical Society 1948 70
(11),3666-3667 DOI: 10.1021/ja01191a036



