On remark: just because activation energy high/low it doesn't necessarily mean that reaction is slow or fast. You cannot tell this without the pre-exponential factor.
The answer: Off course, the original statement should not applied as you do it.
"According to transition state theory, rates are determined by the rate-limiting step." doesn't mean automatically that all the reaction rates are equal.
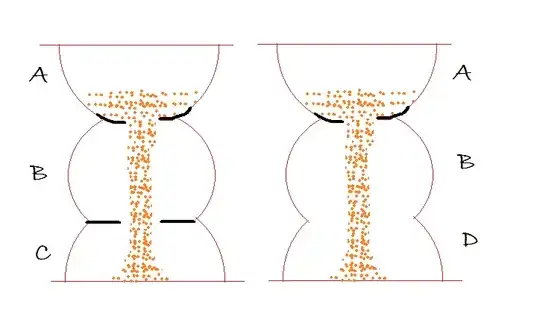
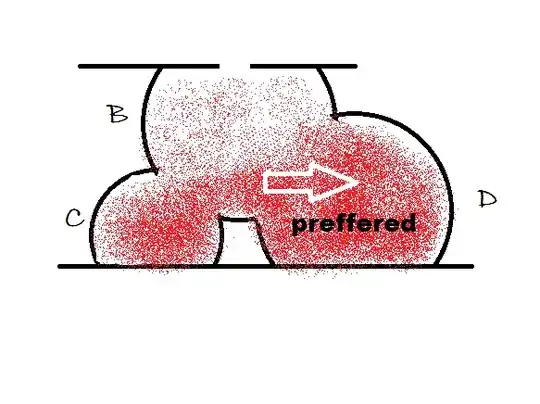
A to B conversion is slow compared to other processes, is true, but still B to C and B to D are competing processes. The original statement only means that B will not build up in bigger quantities, so during the reaction all the final products (C and D together) are approximately equal with the used A after you consider stoichimetries.
Edited for clarification (sorry, pretty new to LaTex):
You should consider all the transformations as independent equations. Black magic is not involved, "rate determining step" and such only helps making approximations so one can solve the coupled differential equations on napkin.
Example 1.
Let us assume a reaction system with $A \to B \to C$. The kinetics of the system can be describe by two independent equations
$A \to B$ with a corresponding $k1$ and $B \to C$ with a corresponding $k2$
To describe conversions we can get different equations:
$$ \frac{d[A]}{dt} = -k1 [A] $$
$$ \frac{d[B]}{dt} = k1 [A] - k2 [B]$$
$$ \frac{d[C]}{dt} = k2 [B] $$
We see that it is already pretty messy, so assumptions like $k1 << k2$ can help. The general way is that we say, this difference in the rate constants allows us to asume $$ \frac{d[B]}{dt} \approx 0 $$. So we have pipeline, and in first approximation have that much $B \to C$ conversion going as $A \to B$. I.e. the is no "clogging" in the middle. Not much magic here.
Example 2.
Now what if we have e.g. two slow reactions as second step instead of one? Noting special:
$A\to B$ , with $k1$,
$B\to C$ , with $k2$,
$B\to D$ , with $k3$
which translates to
$$ \frac{d[A]}{dt} = -k1 [A] $$
$$ \frac{d[B]}{dt} = k1 [A] -( k2 [B] + k3 [B])$$
$$ \frac{d[C]}{dt} = k2 [B] $$
$$ \frac{d[D]}{dt} = k3 [B] $$
Now we have the same assumption that the first step is much slower than the others than
$$ \frac{d[B]}{dt} = k1 [A] -( k2 [B] + k3 [B]) \approx 0$$
I think you can try it from here and see how the rates comes out.