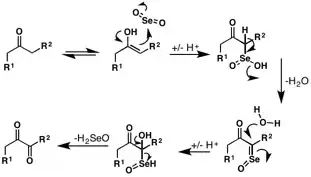
I have searched the whole internet but I could only see examples where the authors of the article took $\ce{R1}$ and $\ce{R2}$ as the alkyl groups around the carbon.
This is regarding the oxidation of carbonyl compounds (I am specifically searching about ketones) using $\ce{SeO2}$.
What I understood was $\ce{C=O}$ got introduced on the alpha-carbon and two hydrogens from that carbon were lost.
I would like to know the product obtained on reacting pentan-2-one with $\ce{SeO2}$. Will the carbon having 3 alpha-hydrogens become $\ce{C=O}$ or will the other one having 2 alpha hydrogens become $\ce{C=O}$?
References:
- Selenium dioxide oxidation of ketones and aldehydes. Evidence for the intermediacy of .beta.-ketoseleninic, K. Barry Sharpless and Kenneth M. Gordon Journal of the American Chemical Society, 1976, 98 (1), 300-301 DOI: 10.1021/ja00417a083
- https://www.adichemistry.com/organic/organicreagents/seo2/selenium-dioxide-seo2.html