None of the US coins are magnetic (ferromagnetic), except for the 1943 Lincoln penny (Steel Cents, made in steel and zinc to save copper for ammunition during wartime), which are considered magnetic. Almost all of those coins other than Steel Cents are made with higher percentage of copper ($\ce{Cu}$) and lower percentages of other metals such as nickel ($\ce{Ni}$), zinc ($\ce{Zn}$), etc. (mostly $\ce{Ni}$). For example, current 5-Cent US coin (US Nickel) is made of 75% $\ce{Cu}$ and 25% $\ce{Ni}$, as OP noted in the question. You can find some percentages in US coins in this AZO Materials Article.
Pure $\ce{Ni}$ is magnetic at room temperature while $\ce{Cu}$ is not. Yet, why US Nickel is not magnetic?
The magnetic properties of $\ce{Cu/Ni}$ alloys have been studied by several researchers (e.g., Ref.1 and 2), perhaps because their use in alloys goes back at least two thousand years, at the time, the knowledge of the composition of alloy was unknown (Note: the elemental nickel was only discovered relatively late on). According to this research, pure $\ce{Ni}$ is magnetic with its Curie temperature being around $\pu{527 K}$ (Curie temperature or Curie point is where a metal loses its ferromagnetism with elevating temperature). When $\ce{Ni}$ mixes with $\ce{Cu}$ to make an alloy, this Curie temperature decreases with increasing amount of $\ce{Cu}$ (Ref.3):
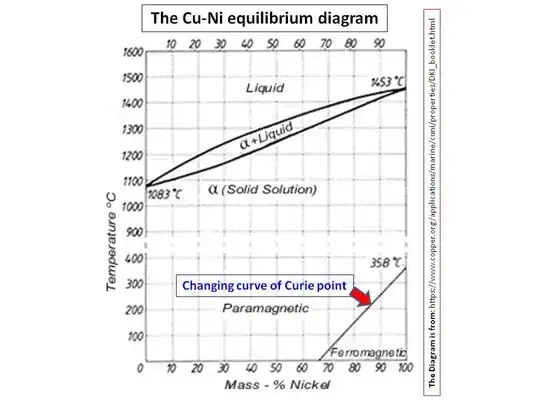
Apparently, the Curie point has become $\pu{0 ^\circ C}$ or lower for $\ce{Cu/Ni}$ alloys with compositions 67:33 or lower $\ce{Ni}$ amount. Thus, it is safe to say alloys with less than 65% $\ce{Ni}$ in $\ce{Cu/Ni}$ alloys do not show magnetic properties at room temperature. Therefore, the US Nickel with 25% $\ce{Ni}$ would not act on permanent magnets.
However, Kaufmann and Starr (Ref.2) have shown that these alloys with less amount of $\ce{Ni}$ would gain their their magnetism at way lower temperatures (about $\pu{14-77 K}$).
References:
- E. H. Williams, "Magnetic Properties of Copper-Nickel Alloys," Phys. Rev. 1931, 38(4), 828 (DOI: https://doi.org/10.1103/PhysRev.38.828).
- A. R. Kaufmann, C. Starr, "Magnetic Properties of Solid Solutions. III. The Paramagnetic Alloys of Copper and Nickel," Phys. Rev. 1943, 63(11-12), 445 (DOI: https://doi.org/10.1103/PhysRev.63.445).
- E. A. Brandes, G. B. Brook, In Smithells Metals Reference Book, Seventh Edition; Butterworth-Heinemann: Woburn, MA, United States, 1992, Chapter 11: Equilibrium Diagrams, pp. 11-236.
