During my study of aromaticity, I came through this topic of quasi-aromaticity. Can you please elaborate what Quasi aromatic compounds are and how are they designated?
Asked
Active
Viewed 5,659 times
16
Mathew Mahindaratne
- 39,943
- 27
- 55
- 107
chail10
- 688
- 6
- 19
-
2Consider using google for such terminology. It has might show good results and is recommended before asking a question. If it is not, then the question is welcome. – user79161 May 24 '19 at 20:32
-
5This appears to be the original paper: Lloyd, D.; Marshall, D. R. Quasi-aromatic compounds: a definition, Chem. Ind. (London) 1964, 1760. Also, there is a modern review by Krygowski et al. (PDF). Feel free to improve and narrow-down your question in the meantime. – andselisk May 24 '19 at 21:15
-
7@user79161 I completely disagree. We definitely welcome many questions that could "just be Googled." Many of these questions may bring users to less reputable sites like Yahoo answers. If we build up a repository of easily-Googled questions, we start showing up in Google which really grows our site. And it helps people get an authoritative answer. – Melanie Shebel May 25 '19 at 07:10
-
4@MelanieShebel This site "shows on Google" more then sci. papers, so that point is moot. While the fact that something can be googled isn't a reason to close a question, because like everything here can be, users should still provide some context and introduction into the topic in the body of question, otherwise downvotes are are proper reaction. – Mithoron May 25 '19 at 22:15
1 Answers
16
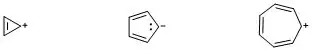
In general, we can describe a quasi aromatic compound as a compound, which is ionic in nature with a counter ion, and the $\pi$ electrons in such compounds follow Huckel's rule ($4n+2$).
In other words, quasi aromatic compounds are those in which the charges present on the molecule are a part of aromaticity of the compound. A few examples of such compounds are depicted in the diagram:
However, it has a deeper and broader meaning. For a more in-depth explanation, please read the given references.
References:
- T. M. Krygowski, B. Bankiewicz, Z. Czarnocki, M. Palusiak, “Quasi-aromaticity—what does it mean?” Tetrahedron 2015, 71(30), 4895–4908 (https://doi.org/10.1016/j.tet.2015.05.074).
- E. Kleinpeter, A. Koch, “Characterization and quantification of quasi-aromaticity by spatial magnetic properties (TSNMRS),” Tetrahedron 2015, 71(33), 5275–5284 (https://doi.org/10.1016/j.tet.2015.06.019).
Melanie Shebel
- 6,704
- 10
- 45
- 86
Mathew Mahindaratne
- 39,943
- 27
- 55
- 107
-
1The references do not seem to match the answer, as the former tend to emphasize secondary bonding as part of the quasi-aromatic structure and the species do not seem to be charged. What am I missing? – Oscar Lanzi May 27 '19 at 01:09
-
@ Oscar Lanzi: I don't think you are missing anything. I think chemists are giving broader picture to existing simple definition. All are still a theory. – Mathew Mahindaratne May 27 '19 at 20:55
-
2@Melanie Shebel: Thank you for careful (and ruthless! :-)) editing. I'll also take an advantage to congratulate you for your new moderator ship! – Mathew Mahindaratne May 27 '19 at 20:58
-
1Even though the gold book calls it Hückel (4n + 2) rule, there are more components to aromaticity than a simple number. Yet it appears that all that ever is taught about aromatic compounds is that number, without any limitations of the generalisation and approximation of these rules. I think it is more important to understand that all compounds that follow Hückel's rules have aromatic character, but not all compounds where aromaticity is observed follow Hückel's rules. – Martin - マーチン May 27 '19 at 21:47