What is the role of amalgamated zinc in the Clemmensen reduction mechanism?
-
Formation of Radical? https://chemistry.stackexchange.com/a/74861/69977 – William R. Ebenezer Apr 08 '19 at 06:24
-
2Zinc just grabbed out of a bottle will have a thin oxide passivation layer. Amalgamating the zinc removes that layer. – MaxW Apr 08 '19 at 06:24
-
And yes, like MaxW said, it is amalgamated to allow it to react to the maximum extent. – William R. Ebenezer Apr 08 '19 at 06:27
-
A topic for a good another question is, how mercury deals with oxide layers. – Poutnik Apr 08 '19 at 06:56
1 Answers
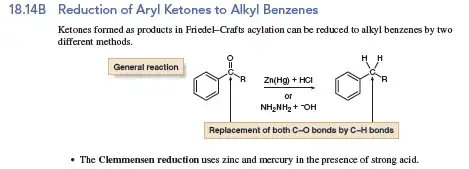
As the illustration below would show, you can use either amalgamated zinc (in a Clemmensen reduction) or hydrazine (in a Wolf-Kishner reduction). This reaction as drawn hides some of the secondary products which should be zinc peroxide and mercury oxide*
Metallic zinc is “activated” by mercury by “dissolving” it into individual atoms of zinc that are distributed evenly in the alloy. You're basically creating a zinc solution.
The reaction of zinc with HCl releases hydrogen gas. However, when this reaction takes place with zinc amalgam, the individual hydrogen molecules that are formed by the reaction of zinc with HCl remain in a reactive state (sometimes called “nascent hydrogen”) and reacts with the ketone to initiate the reduction reaction.
So the mercury in zinc amalgam serves to “trap” the active hydrogen as it is formed, and allows it to attack the carbonyl compound rather than be released as a gas.
*Disclaimer: I have never actually performed this reaction and I could be dead wrong.
- 29
- 4
-
This does not really answer the question of "what is the role of the zinc/merury amalgam". Yes, it is the reductant but why use it in preference to zinc? – Waylander Apr 08 '19 at 09:47
-
I did not understand the question like that, as the requester didn't ask for an explanation of the differences, however I've updated my answer. – Simple Romanian Apr 08 '19 at 10:13
-
I have found an interesting note in the Czech version of the Wikipedia article, that while the amalgam is preferred for alkylarylketones, for dialkylketones is preferred metallic zinc. – Poutnik Apr 08 '19 at 10:53