I have recently started reading about colour in organic molecules and come across conjugation of pi bonds. My question is pretty short... In transition metal ions I understand colour is caused by molecules jumping between the d sub orbitals as a result of ligand splitting them. The concept of excitation easily applies here. So what I want to know is. How do electrons get excited in a conjugated molecule?
-
Maybe a look at this question helps. – Philipp May 11 '14 at 20:18
2 Answers
This first picture shows what happens to the molecular orbitals of ethylene if we bring two ethylene molecules (the right and left sides of the molecular orbital vs. energy part of the diagram) close together and form 1,3 butadiene (center of the figure)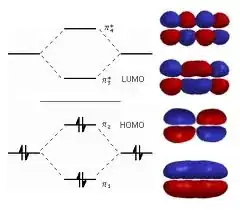
The next figure shows the result of adding a third ethylenic group to the molecule and form 1,3,5-hexatriene. Note how in each case the molecular orbitals combine (split) and generate a new set of molecular orbitals; and in each case the separation between the Highest Occupied Molecular Orbital (HOMO) and the Lowest Unoccupied Molecular Orbital (LUMO) decreases. When light is absorbed an electron is promoted from the HOMO to the LUMO. If we make the HOMO-LUMO separation small enough by extending conjugation, we can shift absorbtion into the visible region (longer wavelength, smaller HOMO-LUMO energy separation). We can add other groups too, like cyano, nitro, phenyl, carbonyl, etc. to extend the conjugation in our system.

- 84,691
- 13
- 231
- 320
To be able to show some color, the photons with wave length within visible light region have to be absorbed as the energy to make electron jump from lower orbital to higher orbital.
In the case of conjugated organic compound, electrons jumps between $\pi$ and $\pi^{*}$ molecular orbitals happen to be within the visible light region. The key is the energy difference, the conjugation system has to be big enough or the energy gap between $\pi$ and $\pi^{*}$ molecular orbitals is too big and the wave length will be too short to be visible.
- 2,098
- 13
- 13