Accommodation in the major or minor groove
I am not in a position to generalize about all drugs that bind to the major groove of DNA, but at least one well-known example, actinomycin D, does so because it intercalates between the base-pairs in the double helix. Although other weak chemical interactions stabilize this binding, much of it is through base stacking with base-pairs. Hence binding requires it be inserted into the major groove in which the base pairs lie (see e.g. PDB 101 Molecule of the Month article).
Binding of calicheamicins, in contrast, involves interaction with two parts of the molecule. The ‘head’ of the molecule makes specific interactions with a TCCT sequence in the DNA (in the case of calicheamicin γ1), which is achieved from the minor groove (intercalation is not necessary) allowing the saccharide ‘tail’ to fit into the minor groove. (Ikemoto et al. 1995).

(Constructed from 3D Structure images on the Protein Data Bank website)
Sequence specificity
A concern of the poster is the fact that the interaction of calicheamicin is sequence specific (there is apparently a preference for d(T-C-C-T).d(A-G-G-A)) whereas access to the bases is restricted in the minor groove. This is addressed in the paper by Ikemoto et al., but without illustration, assuming you are able to visualize the chemical interactions in your mind. I have used Jmol to view the structure 2PIK, have prepared a couple of screen shots to help illustrate the details I shall quote from the text of the paper.
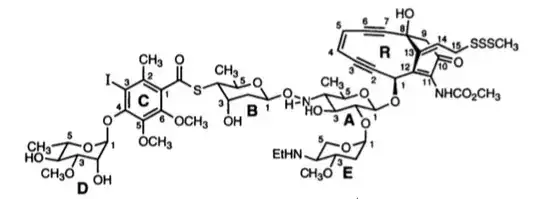
Cleavage of the DNA is performed by the enediyne aglycone (R), which does not make contact with the bases. Two of the regions that do interact with bases are the thio-sugar ring (B) and the aromatic ring (C). These are shown below in the complex of calicheamicin (coloured yellow) with a deoxy-oligonucleotide duplex, together with a close-up of the interactions of these rings.

In the left-hand frame the DNA has standard cpk colouring, and the phosphates (orange–red) lining the minor groove are evident. The base-pairs are perpendicular to the plane of the image. In the second frame I have tilted the image slightly to allow the rings of the bases to be seen, and coloured the bases (actually the whole nucleoside) red/white/green/blue for A/T/G/C. Quoting from the Ikemoto paper:
The thio sugar B is positioned edgewise in the minor groove and contacts the A20 residue through van der Waals and hydrogen bonding (B ring hydroxyl to N3 of the base) interactions. The aromatic ring C is positioned between the walls of the minor groove with its iodine and CH3 groups directed toward the floor of the minor groove.… The S-carbonyl linker, which adopts an orthogonal alignment relative to the plane of ring C (favored by the steric demands of the ortho aromatic ring substituents), bridges the minor groove and makes van der Waals contacts with the opposing walls of the groove.
This may not have the clarity of, say, Watson and Crick base-pairing, but neither does the interaction of proteins with particular DNA sequences — one needs to examine the multiple interactions that occur. What I think it does show is that this drug can make specific contact to bases, even though it binds in the minor groove.


