I don't understand your question either, but my response is to the question in the title. Oxygen has low saturation in blood plasma, so humans require hemoglobin to serve as a transport protein. Oxygen must bind reversibly, otherwise it gets stuck to hemoglobin.
Humans actually have two proteins, hemoglobin and myoglobin. Hemoglobin (HbA) circulates in the blood, whereas myoglobin is in the muscles. Myoglobin (Mb) has a higher affinity for oxygen than hemoglobin (see image). This makes sense, because the hemoglobin in the blood must unload oxygen at the muscles, and myoglobin must bind it. Hemoglobin's affinity for oxygen is regulated by 2,3-BPG, pH, and [CO₂] to ensure that it can unload oxygen at tissues.
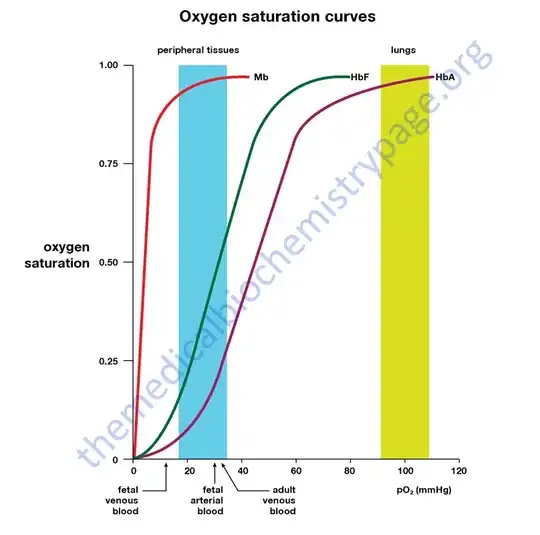 (Curves to the left have higher affinity, meaning that they have a higher oxygen saturation for the same partial pressure of oxygen.)
(Curves to the left have higher affinity, meaning that they have a higher oxygen saturation for the same partial pressure of oxygen.)
Furthermore, humans have several types of hemoglobin. Fetal hemoglobin (HbF) has a higher affinity for oxygen than regular hemoglobin, ensuring that the fetus obtains the oxygen that it needs from the maternal blood.
 (Curves to the left have higher affinity, meaning that they have a higher oxygen saturation for the same partial pressure of oxygen.)
(Curves to the left have higher affinity, meaning that they have a higher oxygen saturation for the same partial pressure of oxygen.)