Summary
The primary metabolic function of aspartate transaminase, like other transaminases is to transfer nitrogen, in the form of amino groups, between 2-oxo acids. These reactions are readily reversible, and so can function in both directions, i.e. in the context of both amino acid synthesis and degradation.
In the liver aspartate transaminase and alanine transaminase are especially important for feeding nitrogen into the urea cycle for disposal as urea. Alanine transaminase transfers amino groups to glutamate, from which ammonium ions are liberated. Carbamoyl phosphate, formed from the ammonia, and aspartate, produced by transamination catalysed by aspartate transaminase, are the nitrogen inputs to the urea cycle. Thus, the poster’s second postulate is correct.
However the poster’s first postulate is incorrect. Aspartate transaminase is not concerned with energy generation, and the demands of the urea cycle ensure that aspartate is normally a product, rather than a substrate, of the transamination in the liver. In certain situations aspartate does feed into the tricarboxylic cycle as oxaloacetate, produced by transamination as shown in the question. This is to replenish oxaloacetate removed for gluconeogenesis, but does not short-circuit the tricarboxylic acid cycle as the α-ketoglutarate consumed in the transamination is subsequently replenished from the glutamate produced.
The central role of glutamate in transamination
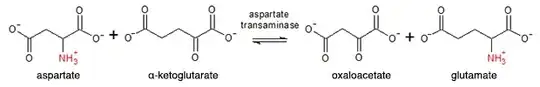
The diagram in the question shows an example of transamination, which is a reversible reaction between two amino acid / keto-acid pairs: the amino group of the first member of the pair replaces the keto group of the second member, with a reciprocal replacement of the amino group of the first member by the keto group of the second. In general this allows the distribution of nitrogen to different carbon skeletons to form a balanced distribution of amino acids for protein biosynthesis.
The poster’s question concerns aspartate transaminase, in which one of the pairs is aspartate/oxaloacetate; but before considering this particular enzyme in the context of the liver, I think it useful to emphasize the central role of the other pair — glutamate/α-ketoglutarate — in the uptake and disposal of ammonia. This is summarized in the diagram below:

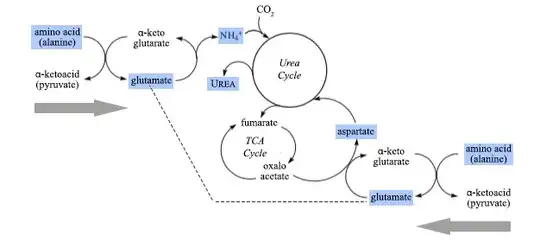
It can be seen that the formation of glutamate from α-ketoglutarate is the entry point of ammonia into metabolism, and that transaminases allow glutamate to transfer its acquired amino group to produce a range of amino acids. In conditions where excess nitrogen must be removed, glutamate is formed from the transamination of α-ketoglutarate by other amino acids, typically with the liberation of ammonia. In aerobic organisms the α-ketoglutarate is available from the pool in the tricarboxylic acid cycle, but is regenerated and so does not drain this pool.
In simple organisms ammonia is released from the cell as such, but in many animals this is not possible and it must be converted to some other nitrogenous compound. In terrestrial mammals this is urea.
The mammalian liver and urea production
There is a cyclic sequence of reactions — the urea cycle — involved in the formation of urea, which occurs in the liver. It is important to realize that the two nitrogens in this compound, (NH2)2CO, come from two different molecular species.
The first is the ammonium ion released by the reduction of glutamate, above, which reacts with carbon dioxide to form carbamoyl phosphate:

The second nitrogen input required for the urea cycle is aspartate, which is also formed from glutamate, but by transferring the amino group of glutamate to oxaloacetate in the aspartate transaminase reaction, but in the reverse direction from that shown in the question:
glutamate + oxaloacetate → aspartate + α-ketoglutarate
At first sight, this formation of aspartate would seem to drain oxaloacetate from the pool of the tricarboxylic acid (TCA) cycle. However the outline diagram below shows that carbon skeleton of aspartate is released from the urea cycle as fumarate. This is an intermediate in the tricarboxylic acid cycle, and regenerates oxaloacetate after conversion to malate.
Alanine transaminase
In the mammalian liver the main transaminase used to transfer amino groups into glutamate is alanine transaminase. This is because glucogenic amino acids produced from protein breakdown in the skeletal muscle are generally converted to alanine before transport to the liver through the blood.
Entry of aspartate into the tricarboxylic acid cycle
The primary role of the TCA cycle is to generate energy by the complete oxidation of the acetyl group of acetyl CoA, derived from fatty acids (especially in the liver) or pyruvate. Oxaloacetate is required for the acetyl group to enter the cycle, and must be regenerated at the end for the cycle to continue. In the liver — especially in starvation — oxaloacetate from the TCA cycle can be converted to phosphoenol pyruvate for gluconeogenesis, providing essential glucose for the brain and nervous tissues (see this answer). This uses amino acids from the breakdown of muscle protein, as mentioned above, and is illustrated by the red arrows in the diagram below:
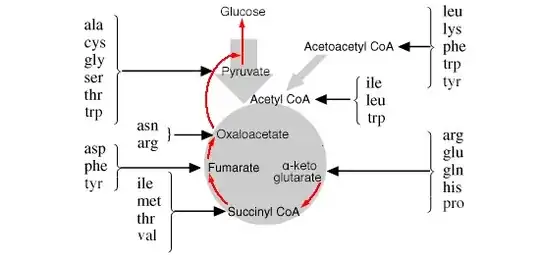
The TCA cycle also serves as a pool of certain intermediates that are starting points for biosynthetic pathways: e.g. oxaloacetate and α-ketoglutarate for the several amino acids, and succinyl CoA for porphyrins. When intermediates are drawn off for this purpose they must be replaced or the TCA cycle will come to a halt, and this is accomplished by input from other compounds such as pyruvate and aspartate, when they are available, in so-called anaplerotic reactions. In the case of aspartate, this is accomplished by the reaction catalysed by aspartate transaminase, in the direction shown in the diagram in the question (as pointed out by @user338907). It should be emphasized that this reaction alone could not maintain the TCA cycle, as it would remove α-ketoglutarate from it. The glutamate produced from this transamination must be converted back to α-ketoglutarate via glutamate dehydrogenase or transamination with some other aminoacid/oxoacid pair. Thus, it would be incorrect to think that α-ketoglutarate is being converted directly to oxaloacetate in these circumstances, forfeiting energy that could be generated from an oxidative conversion in the TCA cycle: the overall reaction is the conversion of (external) aspartate to oxaloacetate + ‘amino group’.
Literature
The topic of transaminases is dealt with in an organism/tissue-agnostic manner in most biochemical texts. I therefore suggest this review of the Urea Cycle that appeared in Biochemistry and Molecular Biology Education, and can be accessed without subscription.