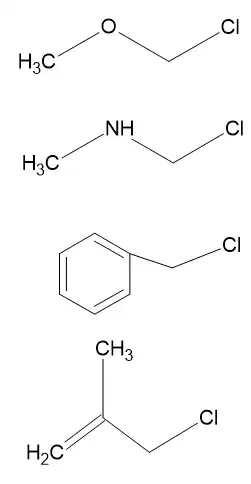
Benzyl and allyl cations are stabilised by the neighbouring $\unicode[Times]{x3C0}$-systems. The electrons in the $\unicode[Times]{x3C0}$-system are actually required for (formally) form double bonds to ensure all-octet structures as well as possible. The cationic structures will contain an electron sextet somewhere (most often drawn on the benzylic carbon for the benzyl cation).
The $\unicode[Times]{x3B1}$-oxygen and $\unicode[Times]{x3B1}$-nitrogen systems on the other hand contain free lone pairs on the heteroatoms. These are not used for double bonds, so they can be shared with the cation to form a new double bond. The resulting mesomeric structure is an all-octet structure which are usually much more stable than non-octet structures (at least for main group elements).
The nitrogen-containing carbocation could even, after the double bond has been formed, loose its proton to create a neutral all-octet structure, the formal $\ce{HCl}$ elimination product.